Achondroplasia added to diseases eligible for state support in Georgia following 19-day protest
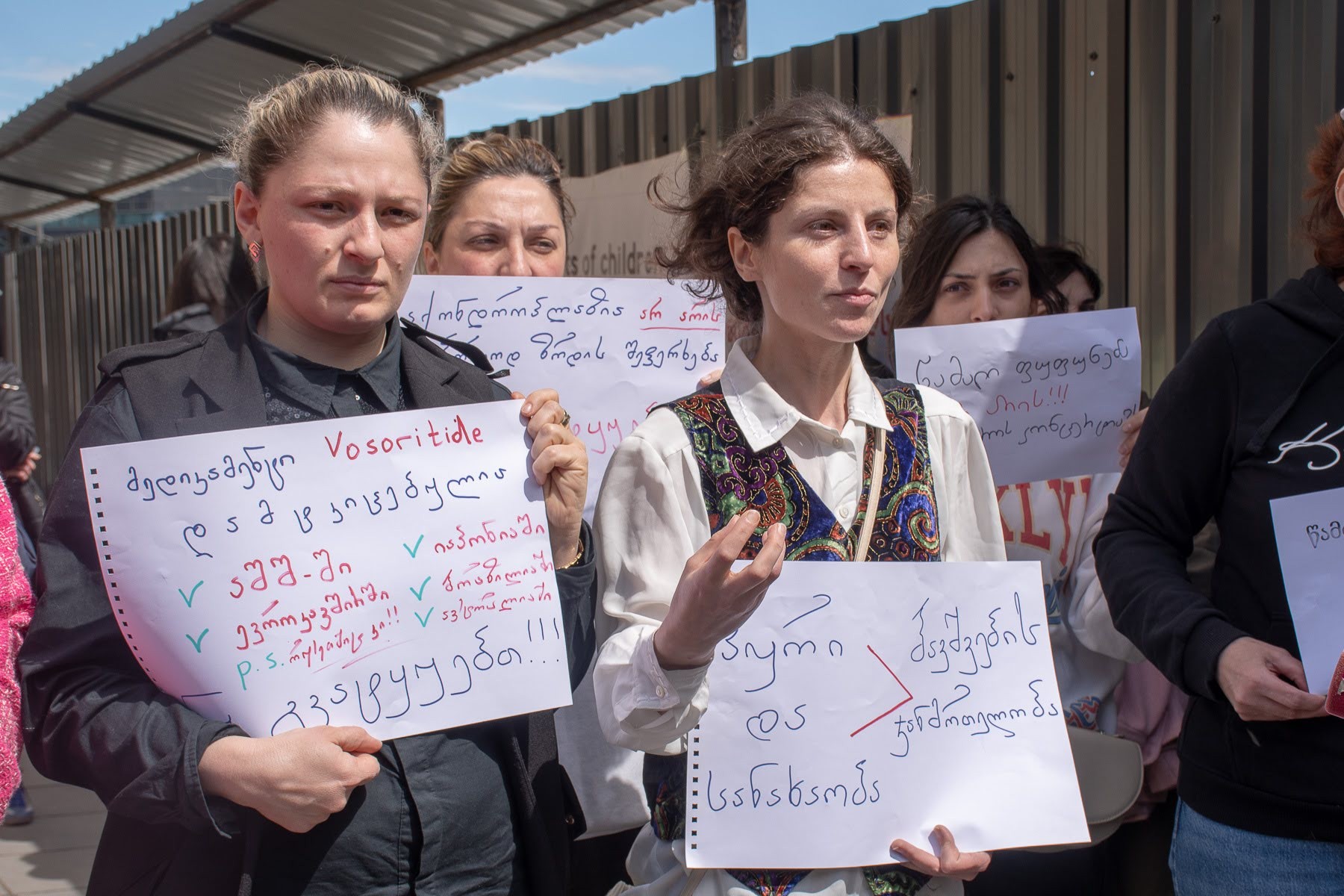
Achondroplasia, a genetic bone growth disorder that causes dwarfism, has been added to the list of rare diseases eligible for state-funded treatment, after parents of children with the condition held a 19-day continuous protest outside the government chancellery.
The amendment to the resolution on state healthcare programmes for 2023, adopted on 30 December 2022, was introduced on 22 May and signed by Prime Minister Irakli Gharibashvili.
A group of parents of children with achondroplasia began their protest outside the Georgian government chancellery on 19 April and remained there until 5 May, at one point blocking a major road leading to Tbilisi’s Freedom Square, to demand that the government import and pay for vosoritide, a drug used to treat children with achondroplasia.
Vosoritide, marketed as VOXZOGO, was approved for medical use in the US and Europe in 2021, and increases growth in children with the condition.
Makuna Gochiashvili, the parent of a child with achondroplasia, wrote that the change meant that ‘the state is taking responsibility for providing treatment […] for people with achondroplasia’.
She stated that ‘all necessary procedures or therapy other than medicine’ would be provided by the state, but that confirmation regarding the provision of medication would come after the protocol was completed at the end of May.
‘Based on what it sounded like in internal conversations, we have no reason to suspect that vosoritide will not be [provided]’, wrote Gochiashvili. ‘But let’s wait for the protocol.’
Achondroplasia is a rare genetic bone growth disorder commonly resulting in disproportionately short stature, excessive outward curvature of the spine, short limbs, and an oversized head.
While official figures are not available, the campaigning parents claim that approximately 30 people in Georgia have achondroplasia, with 12 children potentially benefitting from the drug out of roughly 16–17 minors with the condition in the country.
[Read on OC Media: Parents of children with rare genetic disorder protest lack of medicine]
An ‘experimental’ drug
During the protests, both Prime Minister Irakli Gharibashvili and Health Minister Zurab Azarashvili stated that the medicine was not being imported into Georgia because the drug was ‘experimental’, and had not yet been authorised by the World Health Organisation.
‘If, by giving this medicine, the health condition of these children becomes so complicated that it gets worse, then it will, of course, be our responsibility in this case’, said Gharibashvili, speaking at a session of government on 24 April. ‘We do not want that to happen; we need to wait’.
However, the European Medicines Agency, the agency responsible for evaluating pharmaceutical products used in the European Union, has concluded that the side effects of the drug are ‘manageable’ and that the ‘benefits are greater than its risks’.
The group of parents stopped their 24-hour protests at the Government Chancellery after a meeting at the Ministry of Health on 5 May.
Speaking to Netgazeti, Salome Gabelashvili, who is the parent of a child with achondroplasia, said she was one of the 25 people who participated in that meeting, which included protesting parents, representatives of the Ministries of Finance and Health, representatives of Georgia’s Public Defender’s office and the World Health Organisation, and doctors working on the protocol.
People With Achondroplasia, a Georgian non-profit organisation, wrote on 22 April, that parents of children with achondroplasia had become members of Georgia’s Coordinating Council for the Management of Rare Diseases, in part due to the mediation of Georgia’s Public Defender.
They also noted that Georgia’s Ministry of Finance would allocate funds to purchase Vosoritide when the Ministry of Health approves the drug.




